What exactly is Reverse Osmosis?
Understanding Osmosis First.
To understand what Reverse Osmosis is, we’d better understand first how Osmosis works.
In nature, when two liquid solutions of different concentrations are separated by a semi-permeable membrane, water tends to move through the membrane from the dilute (purer) solution into the more concentrated solution. This natural phenomenon is known as Osmosis. Osmosis happens in all forms of lives. An example of Osmosis in our day-to-day life is the way plants absorb nutrients and water from the soil. Another example is in food preservation when a salty solution is applied to the food. Water leaves the cells of the bacteria (that could spoil the food). As a result, the salt water gets diluted, while the bacteria in the food dehydrate to death.
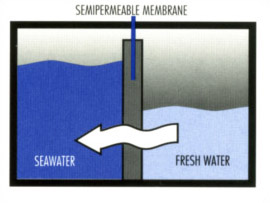
As water molecules permeate through the membrane, pressure on the more dilute side drops, while that on the concentrated solution side rises. Such difference in pressure is called “osmotic pressure differential” of the two solutions. The process of osmosis will continue as long as there is osmotic pressure difference.
May be by now you understand why you can’t drink seawater to kill thirst. You will get killed instead if you do so (because of dehydration).
Reverse Osmosis – Reversing the Osmosis
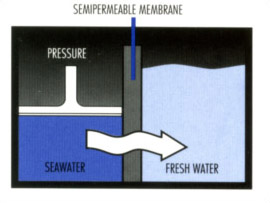
The concept of Reverse Osmosis is simple. By applying sufficient pressure to the concentrate solution (greater than the osmotic pressure difference), the water flow is reversed. Water molecules from the concentrated side are forced through the semi-permeable membrane. In doing so, salts and other dissolved solids are left behind in the more concentrate solution. As a result, a pure water product is obtained from the concentrate solution.
Benefits of Reverse Osmosis
.An effective way to make pure drinking water – by reversing the osmotic process, pure water can be extracted from the concentrate (or contaminated) solution. Because the pores on the separation medium are so small (down to 0.0001 micron) that only water molecules can pass through, RO can be used to remove known contaminants, bacteria, viruses, etc. in water.
.A separation technology rather than a filtration technology – although reverse osmosis (RO) appears to be a filtration process, there are distinct differences between the two technologies. In filtration, the entire liquid stream flows through a porous filter medium, which can be fabric, sand or metal screen. Because the filter medium is porous rather than semi-permeable, it allows the passage of water and dissolved solids (including salts, contaminants, etc.). Particulate matters larger than the pores on the filter medium are trapped on the medium surface. As a result, there is no chemical difference between the water entering the filter (the feed water) and the water exiting after being filtered (the filtrate). Whatever gets dissolved, be they salt, dissolved ions or contaminants, are present still in the water after the filtration process. Reverse Osmosis is very different. The semi-permeable membrane works to separate water molecules from other dissolved solids in the feed water. Thus there is chemical difference between the feed water and the permeate (the pure water product).
.Cross-flow membrane that keeps no unwanted deposits – in reverse osmosis, pressurized feed water flows parallel to the semi-permeable membrane. The pure water molecules of the feed water are able to permeate through the membrane. Particulate matters, bacteria and dissolved solids, which are unable to get through due to their larger sizes, get washed away as concentrate stream. As a result, no unwanted matters or contaminants get deposited on the filter medium. Whereas in conventional filtration, a clogged filter medium can easily become a breeding site of bacteria and pyrogenic matters.
.Energy saving – reverse osmosis requires relatively small amount of energy. Most RO systems can operate at ambient temperature and require electrical power only. In contrast, obtaining pure water using a distillation process requires water to be heated, condensed, then finally collected as fresh water as it cools. In many seawater desalination applications, RO can operate at a fraction of the energy costs of other processes.
References:
1. Letterman, R. D., and Mays, L. W., eds. 1999. Water Quality and Treatment Handbook. 5th ed. Denver: American Water Works Association.
2. Denn, James. 1999. Heterotrophic Menace: fact or fiction? Water Technology, New York. February: 54-62.
3. Paul, David. 1998. Advances continue in membrane technology. Water Technology, New York. March: 98-100.
4. Mukhopadhyay, Deb. 1998. Membranes: crossflow membranes for water recovery at industrial plants. Ultrapure Water, Vol. 15, No. 1, Conn. January: 17-20.
